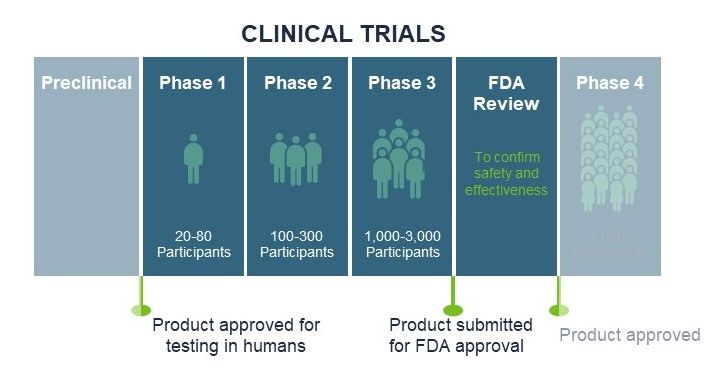
In part two of our three-part webinar series on Risk Management for Your Life Sciences Company, we focus on the risk—especially product liability exposures—for companies conducting human clinical trials.
Life sciences companies face unique exposures and these increase when those companies commence human clinical trials. Based on our recent webinar, this article provides an overview of how you can effectively manage product liability risk as your life science company conducts human clinical trials. We provide tips on global clinical trial product liability insurance programs and the types of insurance policies commonly placed. We include underwriting data requirements, timelines, payment terms, and tips on when to report claims and serious adverse events. As clinical trial insurance programs can be complex to navigate, it is critical that you work with a broker experienced in serving life science companies who has the experience and resources necessary to help you effectively manage the process.

Two types of product liability policies placed by US-domiciled sponsors of human clinical trials include a master policy and, if applicable, foreign local policies.
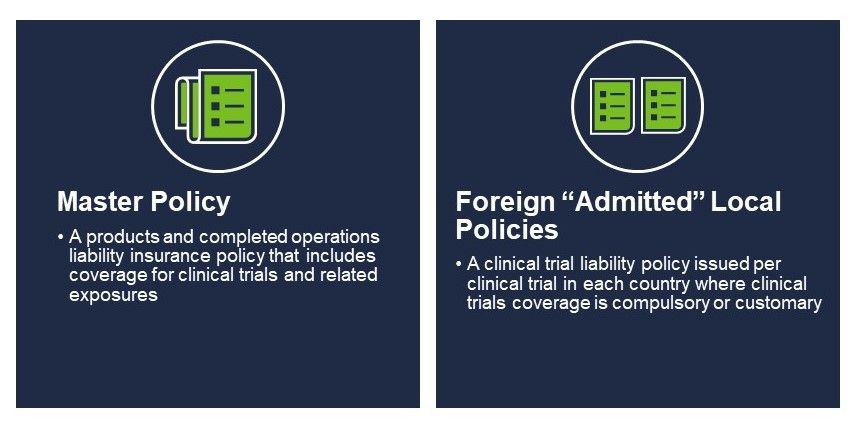
Master policies insure claims alleging bodily injury or property damage arising from the manufacture, handling, or distribution of pharmaceuticals, biologics, or medical devices used in human clinical trials. Policies renew annually for a year and cover all clinical trials conducted by the insured. These policies feature strict claim reporting and defense provisions typical to their “claims-made” policy form. Master policies also require the placement of a run-off or an extended reporting period in the event the insured is acquired, merged into another entity, and/or otherwise ceased operations.
While each program is unique, master policy limits are often set at $5 million for phase I and phase II trials and at $10 million for phase III trials. Available sublimits include medical payments, product recall, and professional liability. The limits may seem lower than you would expect, given humans are involved. However, clinical trials are conducted with strong safety procedures in place and with many protections in place to help mitigate the risk of patient injury.
Typical claim allegations include injury due to clinical trial design defects, failure to warn, and/or manufacturing defects. Standard exclusions include:
The insurance market for product liability insurance is robust, with insurers offering broad coverage terms and conditions at competitive pricing. To secure a quote, you’ll need to submit a completed life science application, financials, clinical trial protocol, and informed consent forms. Once coverage is placed, your broker collects the premium and remits it to the insurance carrier. Your broker also issues certificates of insurance to provide interested third parties with evidence of product liability insurance.
Master policies include a worldwide coverage territory and will respond to covered claims anywhere in the world on a primary basis where legally permitted to do so. The master policy will not respond on a primary basis in those foreign countries where “non-admitted” coverage is prohibited, nor will it satisfy compulsory or customary local clinical trial insurance requirements.
When master policies cannot satisfy foreign local insurance requirements, additional insurance policies may be necessary. This includes countries in most of Europe, Latin America, Asia, parts of Canada and the UK for phase I trials. These policies are issued by local insurance companies licensed to do business in the respective country, are written in the local language, and usually run for the full term of the trial.
Limits are often in local currency in an amount roughly equivalent to $1 million, although certain countries, such as Australia, require significantly higher limits. Companies can place minimum limits required because the master policy responds on an excess basis. While premiums for each individual policy are not especially high, the cost of foreign clinical trial insurance programs can add up when conducting larger trials that include many countries that require local coverage.
In some cases, foreign clinical trial policies include “no-fault” compensation with broad protection for human clinical trial participant injuries. Some countries also require “travel accident” insurance covering the clinical trial participants for injuries sustained traveling to or from their applicable clinical trial site.
Securing foreign “admitted” local policies can be time-consuming depending on the country. As such, start the process a minimum of 60 days prior to the expected ethics committee submission date. While sometimes local policies can be placed in less than two weeks, avoid surprise and potential delays in your clinical trial start date by ensuring the timely submission of insurance documentation.
Companies can secure quotes with basic information for each study, including where it will be conducted, how many participants you plan to screen and enroll, a draft protocol, and target regulatory submission date. Coverage terms, conditions, and information required to bind vary widely by country and even by local insurer. Depending on the country, requirements may include the investigator list, the requirement for applications with original signatures by local legal representatives, official translation of study titles into the local language, payment of local taxes/fees, etc.
The US-domiciled insured is typically allowed to pay the premium for foreign policies and payment is due within 30 days of the date coverage is bound. Prompt premium payment is required regardless of how far in the future the policy is effective. Several countries require pre-payment, referred to as “cash before cover,” before quoting and/or binding coverage. Some countries even require the premium be paid in local currency by local legal representatives.
Local insurers or other third-party intermediaries issue certificates, and this can take much longer than it does when your US broker issues certificates for the master policy. Ask your broker about information needed, realistic timelines, and payment requirements for all potentially applicable countries and plan accordingly.
Foreign policies respond to claims brought in their specific country. To protect against potential gaps in coverage, be sure to report global clinical trial activity to the master policy underwriter and ensure the master policy provides excess and difference in conditions over the foreign “admitted” local policies. Note the master policy will not drop down to provide coverage if there is no local policy in place.
Insurers require prompt reporting of any serious adverse events (SAEs) or claims. In fact, non-disclosure or late notice of these occurrences can prejudice coverage. A claim is defined in the policy but generally considered any written or oral complaints or demands for damages. An SAE is considered a circumstance that could give rise to a claim. Information needed to report claims and SAEs includes the date and location of the event/injury, a brief description of what happened, copies of related correspondence and details of involved third parties.
As clinical trials are unpredictable and evolve quickly, develop a strategy for scaling the product liability insurance program as cost effectively as possible. While you want to provide underwriters a complete picture of the clinical trial your company might start during the policy term, there can be benefits to negotiating certain commitments in the event actual enrollment differs from projections due to routine delays and other clinical trial strategy changes.
Advise your broker of increases or decreases in the projected patient count, new clinical investigator sites added, or updates to the protocol and informed consent forms. Should a trial stop unexpectedly before the policy expiration date, or if no patients are enrolled in a particular country, premium adjustments may be available. Take the time to review your program periodically to ensure it stays in sync with your exposures.